Neogen develops simple tests for shellfish toxins
AYR, Scotland, 25, July 2013 — Neogen Europe Ltd. has added to its comprehensive range of tests for the seafood industry with the introduction of rapid tests to detect the toxins that cause amnesic shellfish poisoning (ASP) and diarrhetic shellfish poisoning (DSP).
Neogen’s new Reveal® 2.0 for ASP detects ASP-causing toxins at a level of 20 parts per million (ppm), and Reveal 2.0 for DSP detects DSP-causing toxins at 160 parts per billion (ppb). Both are one-step rapid tests, and are compatible with U.S. Food and Drug Administration (FDA) and European Union Commission permitted levels.
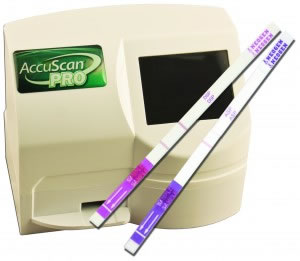
Both new shellfish toxin tests offer easy extraction processes, meaning they are on-site field tests capable of being used on a boat, and are used with Neogen’s innovative AccuScan® Pro Reader that provides consistently accurate and reliable results.
“The combination of the new tests and test reader provide an unparalleled ease in achieving consistently accurate results when testing for these toxins,” said Neogen Europe’s Steve Chambers. “The AccuScan Pro Reader completely eliminates the variance in interpreting test results that can exist when only using a visual appraisal, especially with inexperienced testers. It also provides a very easy method of storing and analysing test results — which is becoming increasingly required as many nations move to reduce the risk posed by these shellfish toxins.”
Toxins that cause ASP are produced by toxigenic diatoms of the genus Pseudo-nitzschia, and primarily include domoic acid (DA). In addition to contamination of seafood, these marine biotoxins can result in human and marine wildlife mortality. The clinical toxicological effects attributed to DA can include: permanent loss of short-term memory, nausea, vomiting, headache, disorientation and loss of balance. Most countries have currently established a maximum permitted level of 20 mg DA per kg whole shellfish (20 ppm).
Toxins that cause DSP include the okadaic acid (OA) group of toxins. OA is produced by marine dinoflagellates such as Dinophysis, and has structural analogs referred to as the dinophysistoxins (DTXs). Clinical toxicological effects attributed to DSP following consumption of contaminated seafood includes diarrhea, nausea and vomiting. The established EU maximum permitted levels are 160 µg OA equivalents (OA, DTXs, pectenotoxins) per kg shellfish meat (160 ppb). FDA action limits are 160 µg (160 ppb) OA equivalents (OA, DTXs) in shellfish. Reveal 2.0 for DSP’s rapid screen is validated to detect OA, DTX1 and DTX2. Due to industry regulations, Neogen can provide an additional validated extraction for the detection of DTX3 in shellfish samples.
Neogen Europe Ltd., the European subsidiary of Neogen Corporation (NASDAQ: NEOG), is a high technology business dedicated to the development and marketing of novel diagnostic kits. These kits focus on topical concerns about the quality and safety of food and agricultural products, from the quality of seed that goes into the ground, right through the chain to the safety of fully processed food products. Neogen Europe was awarded the Queens Award for Enterprise for international trade and development, one of the highest awards bestowed on a UK company.